Omnipod
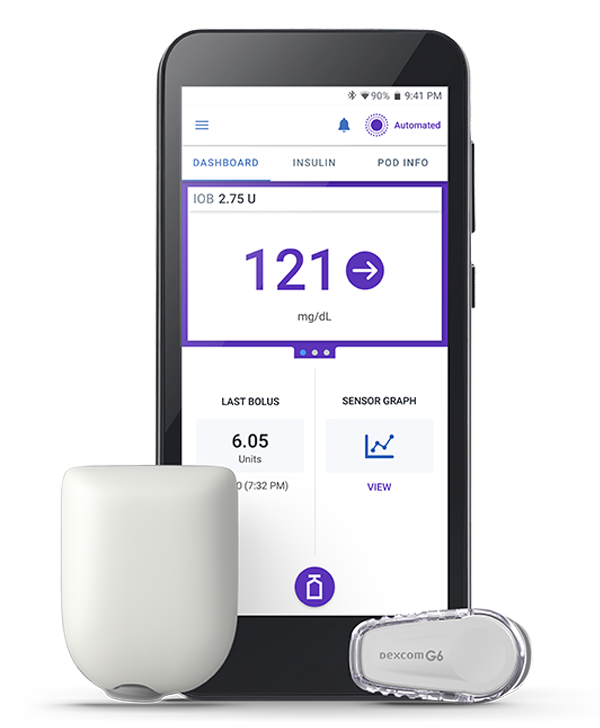
Omnipod 5 Automated Insulin Delivery System
With the Omnipod 5 Automated Insulin Delivery System, your diabetes devices are in sync without tubes to tie you down.† It integrates with the Dexcom G6 Continuous Glucose Monitor (CGM), enabling automatic insulin adjustments to help keep you in range.1,2
You will still need to bolus for meals. This is done with the associated app, either installed on your compatible smartphone‡ or on the Insulet provided Controller.
† Omnipod 5 is cleared for people with type 1 diabetes, ages 2 years and older.
‡ Visit https://www.omnipod.com/current-podders/resources/omnipod-5/device-compatibility for device compatibility

Omnipod DASH® Insulin Management System
A tubeless, wireless insulin management system that lets you experience more freedom with fewer daily hassles.§ Wear the Pod for up to 3 days (72 hours) of continuous insulin delivery, without multiple daily injections. And the convenience doesn’t stop there! Get it all through the pharmacy, with no commitment. Even the Personal Diabetes Manager (PDM) comes at no charge to you with your first prescription.
§ DASH is cleared for anyone with insulin requiring diabetes.
*The Pod has an IP28 rating for up to 25 feet for 60 minutes. The PDM is not waterproof.

Software updates are easy and happen wirelessly

No multiple daily injections when delivering insulin

Easy calculations with an integrated bolus calculator

The pod is waterproof*, just keep the PDM dry

Easy on-the-go insulin delivery without interruption of daily life

The pod is a tubeless insulin delivery system
Check Your Coverage for Omnipod Products*
Get started today with a commitment-free benefits check. Our pharmacy support team will review your coverage and eligibility, so you can see all your options.
Here’s how it works:
-
Fill out the form to get started
-
Once complete, we will reach out to your doctor for a prescription to check your coverage and get back to you within 4-6 business days
———- Frequently Asked Questions ———
The Omnipod is a tubeless, wireless insulin management system that lets you experience more freedom with fewer daily hassles. Wear the Pod for 3 days (up to 72 hours) of continuous insulin delivery, without multiple daily injections.
Omnipod was designed to simplify life with diabetes. From parents struggling to manage a child’s insulin needs, to adults who’ve written off pumps altogether, Omnipod can simplify life™ with 3 days (up to 72 hours) of continuous, tubeless insulin delivery with no multiple daily injections.
Omnipod offers waterproof* concealable insulin management through Pod Therapy. An alternative to traditional insulin pumps, Pod Therapy consists of two primary parts: the tubeless Pod and the handheld Personal Diabetes Manager (PDM), kept nearby to wirelessly program insulin delivery. No multiple daily injections, tubes, or tangles to hold you back.
No, it is not a CGM; it is an insulin management system. Omnipod provides non-stop insulin delivery through a tubeless, waterproof insulin pump called a Pod—all with no multiple daily injections and less daily planning.
Currently, Omnipod 5 is only integrated with the Dexcom G6 CGM. Integration with Dexcom G7 is coming soon. Check out Omnipod’s site for the latest information on Dexcom G7 integration.
Risk Statements:
The Omnipod DASH® Insulin Management System is intended for subcutaneous delivery of insulin at set and variable rates for the management of diabetes mellitus in persons requiring insulin. Additionally, the Omnipod DASH System is interoperable with a compatible blood glucose meter to receive and display blood glucose measurements. The Omnipod DASH System has been tested and found to be safe for use with the following U-100 insulin: NovoLog®, Humalog®, Apidra®, Fiasp®, Lyumjev®, or Admelog®. Refer to the Omnipod DASH Insulin Management System User Guide at omnipod.com for complete safety information including indications, contraindications, warnings, cautions, and instructions.
The Omnipod 5 Automated Insulin Delivery System is indicated for use by individuals with type 1 diabetes mellitus in persons 2 years of age and older. The Omnipod 5 System is intended for single patient, home use and requires a prescription. The Omnipod 5 System is compatible with the following U-100 insulins: NovoRapid®, Humalog®, and Admelog®.The Omnipod 5 Pump (Pod) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The Omnipod 5 Pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. SmartAdjust™ technology is intended for use with compatible integrated continuous glucose monitors (iCGM) and to automatically increase, decrease, and pause delivery of insulin based on current and predicted glucose values. The Omnipod 5 SmartBolus Calculator is intended to calculate a suggested bolus dose based on user-entered carbohydrates, most recent sensor glucose value (or blood glucose reading if using fingerstick), rate of change of the sensor glucose (if applicable), insulin on board (IOB), and programmable correction factor, insulin to carbohydrate ratio, and target glucose value.
WARNING: SmartAdjust technology should NOT be used by anyone under the age of 2 years old. SmartAdjust technology should also NOT be used in people who require less than 5 units of insulin per day as the safety of the technology has not been evaluated in this population.The Omnipod 5 System is NOT recommended for people who are unable to monitor glucose as recommended by their healthcare provider, are unable to maintain contact with their healthcare provider, are unable to use the Omnipod 5 System according to instructions, are taking hydroxyurea as it could lead to falsely elevated CGM values and result in over-delivery of insulin that can lead to severe hypoglycemia, and do NOT have adequate hearing and/or vision to allow recognition of all functions of the Omnipod 5 System, including alerts, alarms, and reminders. Device components including the Pod, CGM transmitter, and CGM sensor must be removed before Magnetic Resonance Imaging (MRI), Computed Tomography (CT) scan, or diathermy treatment. In addition, the Controller and smartphone should be placed outside of the procedure room. Exposure to MRI, CT, or diathermy treatment can damage the components. Visit www.omnipod.com/safety for additional important safety information.
WARNING: DO NOT start to use the Omnipod 5 System or change settings without adequate training and guidance from a healthcare provider. Initiating and adjusting settings incorrectly can result in over-delivery or under-delivery of insulin, which could lead to hypoglycemia or hyperglycemia.

